White Blood Cells in Babies Cerebrsl Spinal Fluid
Types of WBCs
The different types of white blood cells (leukocytes) include neutrophils, basophils, eosinophils, lymphocytes, monocytes, and macrophages.
Learning Objectives
Distinguish between the 2 major types of leukocytes (white blood cells): granulocytes and agranulocytes
Key Takeaways
Primal Points
- The two main types of leukocytes are granulocytes and mononuclear leukocytes (agranulocytes).
- Leukocytes ascend from hemopoietic stem cells in the os marrow.
- Leukocytes are involved in pathogen recognition, phagocytosis (ingestion of particles), pathogen destruction, inflammation mediation, and antigen presentation.
- Granulocytes include neutrophils, basophils, eosinophils, and mast cells. Their granules incorporate enzymes that damage or digest pathogens and release inflammatory mediators into the bloodstream.
- Mononuclear leukocytes include lymphocytes, monocytes, macrophages, and dendritic cells. This grouping is involved in both innate and adaptive immune arrangement role.
Key Terms
- endocytosed: Engulfed during the process by which the plasma membrane of a prison cell folds inwards to ingest material.
- antigen: A substance, usually foreign, that induces an allowed response.
- pathogen: Whatever organism or substance, peculiarly a microorganism, capable of causing disease. Examples include bacteria, viruses, protozoa, or fungi. Microorganisms are non considered pathogenic until the population has grown large enough to cause affliction.
White claret cells (WBCs), or leukocytes, are immune system cells that defend the body against communicable diseases and foreign materials. At that place are several dissimilar types of WBCs. They share commonalities only are singled-out in form and function. WBCs are produced in the bone marrow by hemopoeitic stem cells, which differentiate into either lymphoid or myeloid progenitor cells. A major distinguishing feature is the presence of granules; white blood cells are oft characterized as granulocytes or agranulocytes.
Granulocytes
Granulocytes, too known equally polymorphonuclear (PMN) leukocytes, are characterized by stained granules within their cytoplasm under a microscope. These granules are membrane-bound enzymes that deed primarily in the digestion of endocytosed particles. They may likewise crusade granule dependent cell-mediated apoptosis through the release of perforins, granzymes, and proteases. The nucleus contains multiple lobes (polymorphonuclear) as opposed to a unmarried rounded lobe. Granulocytes contain toll-like receptors that allow them to recognize pathogen-associated molecular patterns (PAMPS). All categories except neutrophils comprise IgE receptors that implicate them in allergic responses. At that place are four types of granulocytes:

Granulocytes: From left to right, a neutrophil, an eosinophil, and a basophil.
- Neutrophils defend against bacterial or fungal infection and other very minor inflammatory processes. They are unremarkably the first responders to microbial infection. Their activity and death in big numbers from degranulation forms purulent necrosis (pus).
- Eosinophils primarily bargain with parasitic infections. They are also the predominant inflammatory cells in allergic reactions.
- Basophils are chiefly responsible for short-term inflammatory response (especially from allergy or irritation) past releasing the chemic histamine, which causes the vasodilation that occurs with inflammation.
- Mast cells role similarly to basophils in that they oft mediate inflammation, but are more than common and ascend from a unlike hemopoeitic lineage.
Mononuclear Leukocytes
Mononuclear (MN) leukocytes are characterized by a unmarried circular nucleus within the cytoplasm. Some MN leukocytes contain granules while others do not, but the members of this group are sometimes considered agranulocytes by naming convention. MN leukocytes comprise lysosomes, small vesicles containing digestive enzymes that suspension down foreign thing that is endocytosed by the cell during phagocytosis. The cells include:
- Lymphocytes, which come in three types. B-lymphocytes produce antibodies in the humoral immune response. T-lymphocytes participate in the jail cell-mediated immune response. NK cells are cytotoxic cells that participate in the innate allowed response past killing virally infected and tumor cells and mediating fever and long-lasting inflammation. B and T lymphocytes contain MHC antigen receptors and their activity is antigen-specific. Other leukocytes will attack any pathogen but cannot distinguish between different types of pathogens.
- Monocytes are large leukocytes that differentiate into macrophages and dendritic cells under varying conditions, while performing like functions in phagocytosis and antigen presentation (the process by which molecular components are presented to lymphocytes to stimulate an adaptive immune response). Monocytes and their progeny contain cost-like receptors and granules.
- Macrophages are monocytes that take migrated out of the blood stream and into the internal body tissues. They destroy necrotic cell debris and foreign textile including viruses and bacteria, and can present antigens to naive lymphocytes. They typically go far at the site of inflammation one to three days subsequently the initial neutrophil response to clean upward expressionless neutrophils, cellular debris, and remaining pathogens.
- Dendritic cells are monocytes that have migrated to cells that are in contact with the external environment, such every bit the skin, intestines, or respiratory epithelium. Their name comes branched projections called dendrites, which increment their surface surface area. They phagocytize pathogens and nowadays antigens to naive lymphocytes.

A Macrophage: A macrophage phagocytizes two smaller particles, possibly pathogens
WBC Function
Each blazon of white claret cell (WBC) has a specific part in defending the body against infections.
Learning Objectives
Depict the functions of leukocytes (white blood cells)
Key Takeaways
Key Points
- Leukocyte functions oftentimes occur in the bloodstream and may represent either the innate or adaptive immune systems.
- Innate immune system functions are non-specific and include phagocytosis, inflammation, and degranulation.
- Adaptive immune system functions are antigen -specific and involve antigen presentation as well as cell -mediated and humoral -mediated activities.
- Compared to innate allowed arrangement functions, adaptive immune system functions have more time to initiate, but piece of work much faster. They take a memory component to prevent reinfection by the same pathogen.
Key Terms
- macrophage: A white blood prison cell that phagocytizes necrotic jail cell debris and foreign material, including viruses, leaner, and tattoo ink. It presents foreign antigens on MHC Ii molecules to lymphocytes. Part of the innate immune system.
- Inflammation: An innate allowed system part in response to a pathogen or injury. Chemical mediators cause the blood vessels to dilate and get more permeable, which draws neutrophils to the area.
- cytotoxic: Any mechanism that can cause the expiry of a cell (typically without phagocytosis), such every bit degranulation or cell mediated apoptosis.
Leukocytes ( white claret cells) provide a number of functions that are primarily related to defending the body from pathogens (foreign invaders). Much leukocyte activeness takes place within the bloodstream, but is not restricted to this area. Many leukocytes are able to perform their functions in tissues or organs during normal transport and in response to injury. Leukocyte functions may exist classified equally either innate or adaptive based on several characteristics.
Innate Immune System Functions
The innate allowed system refers to the body's ability to preclude pathogen entry and destroy pathogens that practise enter the body. Its functions are rapid responses that inhibit a pathogen as presently as it is detected in the body. Innate immune arrangement functions involving leukocytes include:
- Phagocytosis of pathogens. This process is performed primarily by neutrophils, macrophages, and dendritic cells, but about other leukocytes can practise it also. It involves the binding of an Fc receptor to a tail on a pathogen. The pathogen is engulfed by the leukocyte and destroyed with enzymes and free radicals.
- Inflammation. This procedure is performed primarily past mast cells, eosinophils, basophils, and NK cells. When a pathogen is detected or vascular endothelial cells release stress cytokines from injury such as a cutting, leukocytes release a diverseness of inflammatory cytokines such as histamine or TNF-alpha. These cause vasodilation, increase vascular permeability, and promote neutrophil motility to the inflammation site.
- Degranulation. This process is performed past granulocytes similar neutrophils. When pathogens are encountered, granule-dependent apoptosis (a mechanism of cytotoxicity) may be induced in the pathogen by releasing perforins, granzymes, and proteases from their granules.

Neutrophils Phagocytizing Bacteria: Here, neutrophils are depicted phagocytizing and completely engulfing bacteria.
Adaptive Allowed System Functions
The adaptive immune arrangement is specific to each pathogen on the footing of antigens, molecular components of pathogens used by leukocytes to recognize that specific pathogen. Compared to the innate immune system, adaptive immune functions work much faster and have a memory component that prevents reinfection by the same pathogen. Still, more than time typically passes before the adpative immune system is functional. Adaptive allowed functions of leukocytes include:
- Antigen presentation. This process is primarily performed by macrophages and dendritic cells. Following phagocytosis, protein components (antigens) of the pathogen are expressed on leukocyte MHC molecules and presented to naive T cells (and B cells) in the lymph nodes. The T cells will then start the adaptive immune response by rapidly proliferating and differentiating.
- Cell-mediated activities. This process is performed by T cells. Pathogens that bear the T cell's antigen are destroyed through cytotoxic -induced apoptosis and protease activity.
- Humoral activities. This procedure is performed past B cells, which secrete antigen-specific antibodies. The antibodies bind to pathogens to opsonize (mark) them for phagocytes to engulf, neutralize, or start a complement cascade in which proteins form a membrane attack complex to lyse the pathogen.
- Retentiveness cell activity. Following antigen presentation, retentiveness B and T cells are created. These rapidly produce new T cells or antibodies if the same pathogen is detected in the future. This prevents that pathogen from reinfecting the organism.
WBC Formation
Haematopoiesis refers to the formation of blood cells components. It is necessary for vertebrate function.
Learning Objectives
Describe the germination of leukocytes (white claret cells, or WBCs)
Key Takeaways
Fundamental Points
- Haematopoietic stem cells are cocky-renewing and reside in the medulla of the bone ( bone marrow ).
- All blood cells are divided into two chief lineages, produced through lymphoid progenitor cells or myeloid progenitor cells depending on lineage type.
- Lymphoid progenitor cells differentiate into B and T cells and NK cells.
- Myeloid progenitor cells differentiate into myelocytes (granulocytes and monocytes) or non-leukocytes such as erythorocytes and megakaryocytes (which produce platelets).
- Earlier birth, well-nigh claret cell germination occurs in the liver or spleen, which tend to overstate when used for hematopoiesis. In adults, virtually blood production occurs in the bone marrow.
Primal Terms
- myelocyte: A large cell found in bone marrow that becomes a granulocyte or monocyte when mature.
- differentiation: The gradual changes that occur when a jail cell or tissue type changes into a different type. Cells generally become more specialized the more they differentiate, and are considered to be terminally differentiated when they cannot differentiate (and oft cannot carve up) any further.
- megakaryocyte: A large prison cell found in bone marrow, responsible for the production of platelets.
Haematopoiesis refers to the formation of blood cellular components, including both white and blood-red blood cells. All cellular blood components are derived from haematopoietic stem cells located within the bone marrow. In a healthy adult, approximately 1011–1012 new blood cells are produced daily to maintain equilibrium levels in peripheral apportionment.
Leukocyte Haematopoiesis
Haematopoietic stem cells (HSCs) reside in the os marrow and have the unique ability to give rise to all mature blood prison cell types through differentiation into other progenitor cells. HSCs are cocky-renewing. When they proliferate, at least some daughter cells remain HSCs, so the pool of stalk cells does not become depleted over time. The daughters are the myeloid and lymphoid progenitor cells, which cannot cocky renew but differentiate into various myeloid leukocytes and lymphocytes respectively. This is one of the body's vital processes.
Leukocyte Lineages
Two different leukocyte lineages and two not-leukocyte lineages arise from the progeny of HSCs. Post-obit this divide in differentiation, the subtypes undergo eventual differentiation into terminally-differentiated leukocytes, which typically practise not carve up independently.
- The lymphocyte lineage derives from common lymphoid progenitor cells, which in turn become lymphoblasts before differentiating into T cells, B cells, and NK cells.
- Myelocytes are an offshoot of common myeloid progenitor cells, which too differentiate into the erythropoietic and magakaryotic progenitors. This diverse group differentiates into granulocytes and monocytes. Monocytes further differentiate into macrophages or dendritic cells upon reaching certain tissues.
- Megakaryocytes (the cells that produce platelets) and erythrocytes (red claret cells) are non formally considered to exist leukocytes, simply arise from the common myeloid progenitor cells that produce the other cellular components of blood.
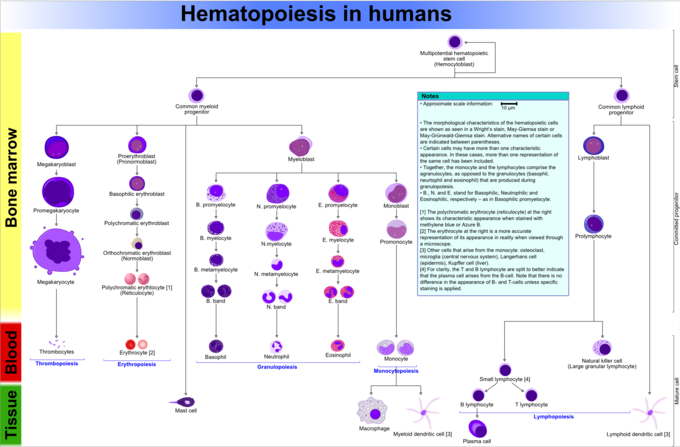
Hematopoiesis in Humans: This diagram shows hematopoiesis as it occurs in humans.
Sites of Haematopoesis in Pre- and Postnatal Periods
In developing embryos, blood formation occurs in aggregates of blood cells in the yolk sac called blood islands. However, most of blood supply comes from the female parent through the placenta. As evolution progresses, blood germination occurs primarily in the spleen, liver, and lymph nodes.
When bone marrow develops, it eventually assumes the job of forming most of the blood cells for the entire organism. Nonetheless, maturation, activation, and some proliferation of lymphoid cells occurs in lymphoid organs (spleen, thymus, and lymph nodes). In children, haematopoiesis occurs in the marrow of the long bones such as the femur and tibia. In adults, it occurs mainly in the pelvis, cranium, vertebrae, and sternum.
In some cases, the liver, thymus, and spleen may resume their haematopoietic office if necessary. This is called extramedullary haematopoiesis. Information technology may cause these organs to hypertrophy and increment in size substantially. During fetal evolution, the liver functions equally the primary haematopoetic organ since basic and marrow develop later. Therefore, the liver is enlarged during development relative to its mature proportions.
lanningbansespoll1947.blogspot.com
Source: https://courses.lumenlearning.com/boundless-ap/chapter/white-blood-cells/
0 Response to "White Blood Cells in Babies Cerebrsl Spinal Fluid"
Post a Comment